
Double nomenclature caused more and more confusion over time.

The name columbium (symbol Cb) remained in use predominantly in American journals. However, instead of replacing one name with the other, the scientific world of that time adopted both names which were used interchangeably.
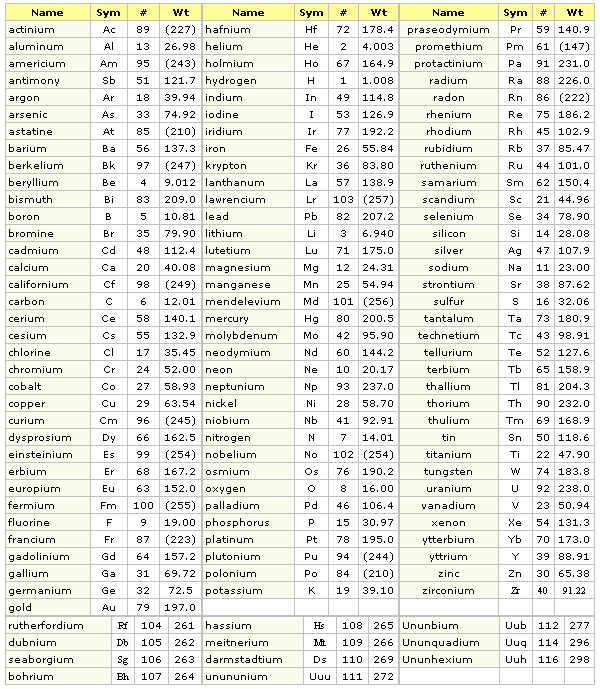
#A LIST OF CHEMICAL ELEMENTS SERIES#
During the next two decades the series of studies clarified that niobium and columbium were one and the same element. As it shortly occured, “pelopium” was a mixture of niobium and tantalum. Thus, the name niobium appeared for the first time in 1844 in the Rose’s work entitled Ueber die Zusammensetzung der Tantalite und ein im Tantalite von Baiern enthaltenes neues Metall, published in Annalen der Physik. Therefore, when the German chemist Heinrich Rose in 1844 published his studies on the tantalum ores, he did not receive any major objections to propose names “niobium” and “pelopium” for the suggested new elements in the ore without looking back at previous research (Rose 1844). In a way this critical paper nullified the studies of the composition of columbite. In 1809 William Hyde Wollaston questioned the existence of columbium as a different element from tantalum (Wollaston 1809). Both the new element and the mineral were named in the same manner “columbium” and “columbite” respectively, reflecting that the specimen of the ore came from America (Columbia) (Wisniak 2015). In short: a new element was discovered in 1801 by Charles Hatchet in the mineral found in the British Museum (Hatchet 1802). Naming of niobium had a complex and somewhat unusual history. For over forty years tantalum and niobium were thought to be one and the same as they share very similar chemical and physical properties and are always found together in nature (Baccolo 2015). Tantalum was first extracted from mineral samples and described by Anders Ekeberg in his work presented in the journal Kungliga Svenska Vetenskapsakademiens Handlingar in 1802, in the article entitled Uplysning om ytter jordens egenskaper, i synnerhet i jämförelse med berylljorden: om de fossilier, hvari förstnämnde jord innehålles, samt om en ny uptäckt kropp af metallisk natur (Ekeberg 1802). The article also highlights some of the more spectacular and important "blind alleys" in the history of science, as well as prosaic mishaps and coincidences during the discoveries that happened to nineteenth-century researchers in the race for the laurel of the first discoverer. Another milestone in the discovery of elements was the application of a spectrometer that allowed the discovery of new elements only on the basis of their electromagnetic spectrum. This fact allowed the scientists to search more systematically. It was a turning point in the development of chemistry, making it possible to predict the existence and properties of yet undiscovered elements. A kind of race began in which the framework of the rules was established only by Dmitri Mendeleev in 1869 with the formulation of the law of periodicity of chemical elements. In the nineteenth century, discoveries accelerated, mainly due to the intensive development of analytical and physical chemistry. By the end of the eighteenth century 37 chemical elements had been described and named.


 0 kommentar(er)
0 kommentar(er)
